Hearing Loss and Aging Implications for Audiologists
November 2014
Pamela Souza, PhD, CCC-A
Our population is getting older. Much older. In the 2010 census, nearly 1 in 5 adults in the United States was over 60 years of age. Over the next 30 years (the likely career span of an audiologist), the percentage of the population over 65 is expected to double, and the population over 85 is expected to triple. Older adults are not only living longer, but working longer and staying socially active later in life. In other words, their communication needs are greater than ever. The rate at which these adults seek help is likely to increase, driven by their communication needs and by improved access to health care. Older adults' comfort level with technology is also increasing (Wandke, Sengpiel, & Sönksen, 2012). Most clinical audiologists have long served the aging population, but are now poised for a transition in what aging means to audiology practice.
Age-related hearing loss is not inevitable, but it is a common consequence of aging. Presbycusis (age-related hearing loss) may be coupled with noise exposure, illness, or use of ototoxic medications that have occurred over many years of life. Recent studies recognize an association between hearing loss and diabetes (Horikawa et al., 2013) and between hearing loss and vascular disease (Lee, 2013), each of which occurs at much higher rates in older adults. Even using relatively narrow definitions, we can estimate that hearing loss affects up to 40% of adults over 65 years, 60% of adults over 75, and 80% of adults over 85 years (Cruickshanks, Tweed, Wiley, Klein, Chappell, et al., 2003; Gates, Cooper, Kannel, & Miller, 1990; Reuben, Walsh, Moore, Damesyn, & Greendale, 1998). Simply put, the majority of older adults have some hearing difficulty, and the consequences of that difficulty can be substantial. Hearing loss is associated with depression (Gopinath et al. 2012; Savikko, Routasalo, Tilvis, Strangberg, & Pitkala, 2005), reduced employment prospects (Kyle & Wood, 1985), and family stress (Scarinci, Worrall, & Hickson, 2008). Due to their appointment constraints and training, primary care physicians may play a minimal role in hearing loss identification and treatment. Audiologists connect the patient to support, information, and appropriate rehabilitative options. Because good communication requires cooperation from both talker and listener, audiologists also provide community education, especially in regard to hearing and accommodations in public spaces. To provide full-service treatment, audiologists must understand the context of age-related hearing loss.
Healthy Aging and Cognitive Changes
Age, as they say, brings wisdom, and many cognitive abilities are honed throughout our lives. Wisdom and creativity continue to the end of life. Daily occupational and social functioning is not impaired by normal aging. Most aspects of language ability remain strong, and some (such as the ability to draw information from context) offer compensatory choices that can offset other declines (Pichora-Fuller, 2008). However, even healthy aging is often accompanied by cognitive changes. Long-term memory may decline. The rate at which new information is learned can be slower, and older adults often have a greater need for repetition of new information. Examples of specific cognitive abilities important to auditory communication include processing speed, working memory, and executive function. Each of these abilities is necessary to communication.
Processing speed allows the listener to take in, evaluate, and assign meaning to a rapidly changing acoustic signal. Because the typical speech rate is approximately four syllables per second, listeners are taking in more than 240 chunks of phonemic information per minute. While processing lags, the conversation has moved on, and older listeners with hearing loss sometimes report feeling fatigued with attempts to keep up.
Working memory allows simultaneous processing and storage during a listening (or reading) task and is essential to assigning meaning to what is heard. Working memory is likely to be required to a greater extent when the acoustic signal is degraded (Rönnberg, Lunner, Zekveld, Sörqvist, Danielsson, et al., 2013), as occurs with background noise or reverberation, or when the listener has hearing impairment. In that situation, the acoustic (and/or visual) input may be ambiguous, requiring the listener to compare the input to choices within the mental lexicon where meaning is assigned. A similar problem has been shown for patients with low working memory who receive complex hearing aid processing that significantly alters signal acoustics, probably because the resulting signal is less familiar (and therefore less unconsciously processed; Arehart, Souza, Baca, & Kates, 2013; Lunner, 2003). Taken as a whole, results of working memory studies suggest that listeners with low working memory may perform best with low-alteration processing that employs a slow wide-dynamic range compressor and minimal amounts of frequency compression, provided there is good signal fidelity.
Finally, executive function allows the listener to direct attention and to ignore extraneous information, an important skill for listening to a target talker in a background of other talkers or for switching attention among talkers. In population studies, all three abilities decline somewhat with age. Importantly, there is also variability among individuals.
Although tests are available to evaluate specific cognitive abilities, such testing is not yet a common feature of an audiometric evaluation. In general, one might expect an 80-year-old patient to process information more slowly, have somewhat lower working memory, and perhaps have less ability to ignore distracting auditory information than a 40-year-old. In caring for older patients, one should consider how cognitive ability affects communication and learning. This is not to suggest that older patients should be patronized, but rather that the appointment structure and supporting materials should be designed to accommodate the entire range of abilities in older patients.
Aging and Dementia
An unfortunate consequence of aging is an increased risk of dementia. New guidelines from the Alzheimer's Association (Sperling, Aisen, Beckett, Bennett, Craft, et al., 2011) present three stages of pathological cognitive change.
- It is theorized that the initial stage is a pre-clinical stage, in which physiological changes have occurred, but significant behavioral symptoms are not apparent.
- Mild cognitive impairment (MCI) presents with memory loss or changes to thought processes that are greater than those that occur with typical aging, but throughout which the patient maintains independence. It is not a foregone conclusion that MCI will progress to dementia, although that is a possibility (Bensadon & Odenheimer, 2013).
- In Alzheimer's dementia, memory loss, changes in behavior, impaired judgment, and difficulty processing visual/spatial information are significant and will impair independence. Later symptoms of the disease (albeit less likely to be seen by clinical audiologists) include difficulty speaking, swallowing, and walking.
Less common causes of dementia (still affecting large numbers of individuals) include dementias caused by vascular incidents, dementia with Lewy bodies, and Parkinson's disease dementia. The prevalence of dementia is estimated at nearly 15% of adults over 70 years of age, with higher incidence in older age groups [PDF]. Considering the large numbers of adults affected, audiologists are very likely to see patients at least with MCI and, often, with dementia as well.
While patients in late-stage dementia and their families are under a significant burden and hearing care may not be a priority, improved communication is an important goal throughout the stages of the disease. Inability to hear may result in new or exaggerated symptoms that are mistakenly attributed to cognitive decline, including failure to respond or responding inappropriately to questions. Amplification (via a hearing aid with automatic function, if appropriate, or other assistive device) can improve communication (Petitot, Perrot, Collet, & Bonnefoy, 2007; Durrant, Palmer, & Lunner, 2005) and may reduce cognitive load. Physicians, speech-language pathologists, and occupational therapists who work with patients with cognitive disorders should be alert to a possible need for amplification and refer appropriately.
Does Hearing Loss Increase Dementia Risk?
Over the past few years, some compelling epidemiology data have shown that hearing loss is associated with increased risk of cognitive decline (Lin, Yaffee, Xiz, Zue, Harris, et al., 2013) and/or dementia (Lin, Metter, O'Brien, Resnick, Zonderman et al., 2011; Gurgel, Ward, Schwartz, Norton, Foster, et al., 2014). The association persists even after controlling for hearing-related systemic conditions, such as vascular or cardiac disease. The more hearing loss the patient has, the stronger the association.
The puzzle is the source (or sources) of the association. First, there might be some underlying physiological or neurological change resulting in both cognitive change and hearing loss. Although major diagnoses were considered (and adjusted for) as confounding factors in those studies, that did not preclude more subtle systemic effects, such as microvascular dysfunction. Second, we know that social isolation is associated with increased risk of dementia (Holwerda, Deeg, Beekman, van Tilburg, Stek, et al., 2012). If hearing loss leads to withdrawal from social and family activities, the isolation itself might be the precipitating factor. Third, hearing loss and loss of signal redundancy require that the patient compensate through effortful listening. Such effort might deplete cognitive resources, leading to a decline. We also have evidence that brain volume changes as a result of hearing loss (Lin, Ferrucci, An, Goh, Doshi, et al., 2014). For any given patient, the increased risk is relatively small. But in a population, the issue presents a possible public health opportunity: Can early correction of hearing loss improve the incidence of dementia? Continued research may help untangle these factors.
The Audiologist's Role in Screening for Cognitive Impairment
A minority of clinics provide formal screening for cognitive function as part of their test battery. Simple cognitive screening metrics are readily available, and patients of concern can be referred to their physician for follow-up. However, in some facilities, clinic time may not permit formal screening, or the audiologist may not feel comfortable with the discussion of specific test results and the concerns regarding memory that might be raised by those tests. An alternative way to consider cognition in audiology practice is simply to be an alert provider. A recent article by Remensnyder (2012) offers a wealth of strategies to consider. Audiologists can be alert to aberrant communication, such as inappropriate affective reactions, or difficulty with memory or word finding. Audiologic history should include questions about memory (while being aware that many normally aging adults will admit to memory concerns), depression, and history of head injury (associated with cognitive change and increased dementia risk). Although many patients will not rise to the level requiring medical follow-up, awareness of memory concerns (and possible MCI) will dictate the extent of supportive audiology care.
Considering Cognition in Hearing Aid Fitting and Training
We can support older patients who are cognitively impaired with a variety of strategies. Family members and communication partners should be encouraged to participate in appointments, both for the patient's benefit and for their own information. Some hearing aid features can ease the burden on the patient. Those features include automatic directivity, automatic program change and telecoil activation, or verbal prompts ("change battery" rather than a signal beep). It can be helpful to disengage all audible alerts, except those considered essential (e.g., battery change) to minimize the need to discriminate and interpret signals.
Because listening in high levels of background noise is most detrimental to listeners with poor working memory, a thoughtful approach to noise reduction is warranted. This should include use of directional microphones and/or assistive devices that improve signal-to-noise ratio and perhaps also use of low-distortion digital noise reduction (Ng, Rudner, Lunner, Pedersen, & Rönnberg, 2013). Care should be taken that assistive technology is not so complex that it prevents ready use.
Clear, brief, written materials (such as a quick reference card outlining the procedure for using the hearing aid for telephone listening or a label on the hearing aid case to prompt daily cleaning) can serve as reminders. More frequent appointments offer a chance to check in with the patient and reinforce new skills. Optional or bundled aural rehabilitation sessions are an excellent option to empower the patient and train him or her on hearing aid use and good communication. Even simple strategies—such as modifying the listening environment—may not be obvious to the patient or family. Review and practice are essential. Remember that much of the information provided at a health-care appointment is forgotten, and at least half of the recalled information is incorrect (Kessels, 2003; McGuire, 1996).
To consider all practicalities, consider how changes in vision and dexterity can impact rehabilitation. Arthritis affects as many as half of adults over 65 years and can make it difficult to feel or manipulate small items, such as hearing aid batteries and controls. Weakening vision reduces reading speed and causes problems seeing in dim light, reading small print, and locating objects. Specific vision impairments, such as cataracts and macular degeneration, may make it impossible to read instruction manuals. Patients may not volunteer this information, either because they aren't prompted to discuss it or because coping with reduced sensation has become a normal (and unremarkable) part of life.
The focused view of the audiology clinic as a place to treat hearing is and will continue to change. Figure 1 shows the complex interrelationship of factors associated with aging that touch clinical care in some way. Older adults comprise ever-larger numbers of an audiologist's patients, and those older adults have unique needs with regard to hearing, cognition, and overall health. Priorities for those adults include health, independence, and coping with adversity (Phelan, Anderson, LaCroix, & Larson, 2004). Age-related cognitive change presents a special challenge. Cognitive changes may affect communication directly—by impairing abilities that are drawn on in difficult listening—or indirectly—by changing the ability to adapt and to engage in social activities. The social milieu may also be different for older adults. Sixty-four percent of older Americans ages 65–74 are married and live with a spouse. After age 85, only 24% live with a spouse, and half live alone. Changes to social support —such as widowhood—can adversely affect health outcomes (Bisconti & Bergeman, 1999). The audiologist can be most effective by considering the patient's lifestyle, needs, interests, and challenges; by listening attentively; and by using that information to provide customized and responsive treatments.
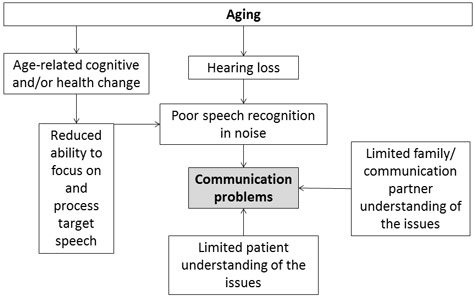
Figure 1
Acknowledgments
The author's work is supported by the National Institute on Deafness and Other Communication Disorders (R01 DC60014 and R01 DC12289). The author thanks Kathryn Arehart, Thomas Lunner, Linda Remensnyder and Jing Shen for helpful conversations regarding aging, cognition, and patient care.
About the Author
Pamela Souza is a professor at Northwestern University. Throughout her career, she has combined academic teaching and research with work as a clinical audiologist. She has worked with patients ranging from pediatric to geriatric populations. She directs a longstanding research program in effects of hearing aids, particularly for older listeners and those with severe hearing loss. Her interests include use of signal-processing amplification, which affects acoustic speech cues, how those changes interact with listener age and cognitive status, and how research findings in this area can direct clinical practice. Dr. Souza is a Fellow of the American Speech-Language-Hearing Association. Contact him at psouza@northwestern.edu.
References
Arehart, K. H., Souza, P., Baca, R., & Kates, J. M. (2013). Working memory, age, and hearing loss: Susceptibility to hearing aid distortion. Ear and Hearing, 34(3), 251–260.
Bensadon, B. A., & Odenheimer, G. L. (2013). Current management decisions in mild cognitive impairment. Clinics in Geriatric Medicine, 29, 847–871.
Bisconti, T. L., & Bergeman, C. S. (1999). Perceived social control as a mediator of the relationships among social support, psychological well-being, and perceived health. Gerontologist, 39, 94–103.
Cruickshanks, K. J., Tweed, T. S., Wiley, T. L., Klein, B. E., Klein, R., Chappell, R., ... Dalton, D. S. (2003). The 5-year incidence and progression of hearing loss: The epidemiology of hearing loss study. Archives of Otolaryngology–Head & Neck Surgery, 129, 1041–1046.
Durrant, J. D., Palmer, C. V., & Lunner, T. (2005). Analysis of counted behaviors in a single-subject design: Modeling of hearing aid intervention in hearing-impaired patients with Alzheimer's disease. International Journal of Audiology, 44, 31–38.
Gates, G. A., Cooper, J. C., Jr., Kannel, W. B., & Miller, N. J. (1990). Hearing in the elderly: The Framingham cohort, 1983–1985. Part I. Basic audiometric test results. Ear and Hearing, 11, 247–256.
Gopinath, B., Hickson, L., Schneider, J., McMahon, C. M., Burlutsky, G., Leeder, S. R., & Mitchell, P. (2012). Hearing-impaired adults are at increased risk of experiencing emotional distress and social engagement restrictions five years later. Age and Ageing, 41, 618–623.
Gurgel, R. K., Ward, P. D., Schwartz, S., Norton, M. C., Foster, N. L., & Tschanz, J. T. (2014). Relationship of hearing loss and dementia: A prospective, population-based study. Otology & Neurootology, 35, 775–781.
Holwerda, T. J., Deeg, D. J., Beekman, A. T., van Tilburg, T. G., Stek, M. L., Jonker, C., & Schoevers, R. A. (2012). Feelings of loneliness, but not social isolation, predict dementia onset: Results from the Amsterdam Study of the Elderly (AMSTEL). Journal of Neurology, Neurosurgery & Psychiatry, 85, 135–42.
Horikawa, C., Kodama, S., Tanaka, S., Fujihara, K., Hirasawa, R., Yachi, Y., ... Sone, H. (2013). Diabetes and risk of hearing impairment in adults: A meta-analysis. Journal of Clinical Endocrinology & Metabolism, 98, 51–58.
Kessels, R. P. C. (2003). Patients' memory for medical information. Journal of the Royal Society of Medicine, 96, 219–222.
Kyle, J., & Wood, P. (1985). Vocational aspects of acquired hearing loss. International Journal of Rehabilitation Research, 8, 425–434.
Lee, K. Y. (2013). Pathophysiology of age-related hearing loss (peripheral and central). Korean Journal of Audiology, 17, 45–49.
Lin, F. R., Ferrucci, L., An, Y., Goh, J. O., Doshi, J., Metter, E. J., ... Resnick, S. M. (2014). Association of hearing impairment with brain volume changes in older adults. Neuroimage, 15, 84–92.
Lin, F. R., Metter, E. J., O'Brien, R. J., Resnick, S. M., Zonderman, A. B., & Ferruci, L. (2011). Hearing loss and incident dementia. Archives of Neurology, 68, 214–220.
Lin, F. R., Yaffe, K., Xia, J., Xue, Q-L., Harris, T. B., Purchase-Helzner, E., ... Simonsick, E.L. (2013). Hearing loss and cognitive decline in older adults. JAMA Internal Medicine, 173, 293–299.
Lunner, T. (2003). Cognitive function in relation to hearing aid use. International Journal of Audiology, 42, S49–S58.
Lunner, T., & Sundewall-Thoren, E. (2007). Interactions between cognition, compression, and listening conditions: Effects on speech-in-noise performance in a two-channel hearing aid. Journal of the American Academy of Audiology, 18, 604–617.
McGuire, L. C. (1996). Remembering what the doctor said: Organization and older adults' memory for medical information. Experimental Aging Research, 22, 403–428.
Ng, E. H., Rudner, M., Lunner, T., Pedersen, M. S., & Rönnberg, J. (2013). Effects of noise and working memory capacity on memory processing of speech for hearing-aid users. International Journal of Audiology, 52, 433–441.
Petitot, C., Perrot, X., Collet, L., & Bonnefoy, M. (2007). Alzheimer's disease, hearing impairment and hearing-aids: A review [Translated from the French]. Psychologie & Neuropsychiatrie du Vieillissement, 5, 121–125.
Phelan, E. A., Anderson, L. A., LaCroix, A. Z., & Larson, E. B. (2004). Older adults' views of "successful aging"—How do they compare with researchers' definitions? Journal of the American Geriatrics Society, 52, 211–216.
Pichora-Fuller, M. K. (2008). Use of supportive context by younger and older adult listeners: Balancing bottom-up and top-down information processing. International Journal of Audiology, 47, S72–S82.
Remensnyder, L. S. (2012). Audiologists as gatekeepers and it's not just for hearing loss. Audiology Today, July/August, 24–31.
Reuben, D. B., Walsh, K., Moore, A. A., Damesyn, M., & Greendale, G. A. (1998). Hearing loss in community-dwelling older persons: National prevalence data and identification using simple questions. Journal of the American Geriatric Society, 46, 1008–1011.
Rönnberg, J., Lunner, T., Zekveld, A., Sörqvist, P., Danielsson, H., Lyxell, B., Dahlström, Ö., ... Rudner, M. (2013). The Ease of Language Understanding (ELU) model: Theoretical, empirical, and clinical advances. Frontiers in Systems Neuroscience, 7, 1–17.
Savikko, N., Routasalo, P., Tilvis, R. S., Strangberg, T. E., & Pitkala, K. H. (2005). Predictors and subjective causes of loneliness in an aged population. Archives of Gerontology and Geriatrics, 41, 228–233.
Scarinci, N., Worrall, L., & Hickson, L. (2008). The effect of hearing impairment in older people on the spouse. International Journal of Audiology, 47, 141–151.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., .... Phelps, C.H. (2011). Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dementia, 7, 280–292.
Wandke, H., Sengpiel, M., & Sönksen, M. (2012). Myths about older people's use of information and communication technology. Gerontology, 58, 564–570.











